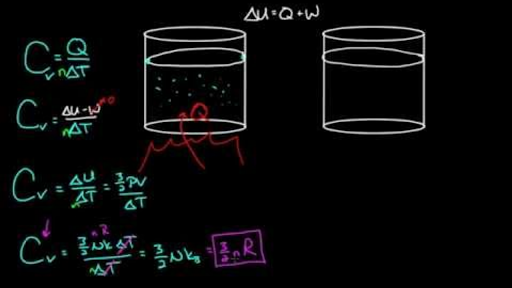
Variation of the constant volume heat capacity C V with the ratio of T... | Download Scientific Diagram

Heat capacity of constant volume C v and constant pressure C P as a... | Download Scientific Diagram
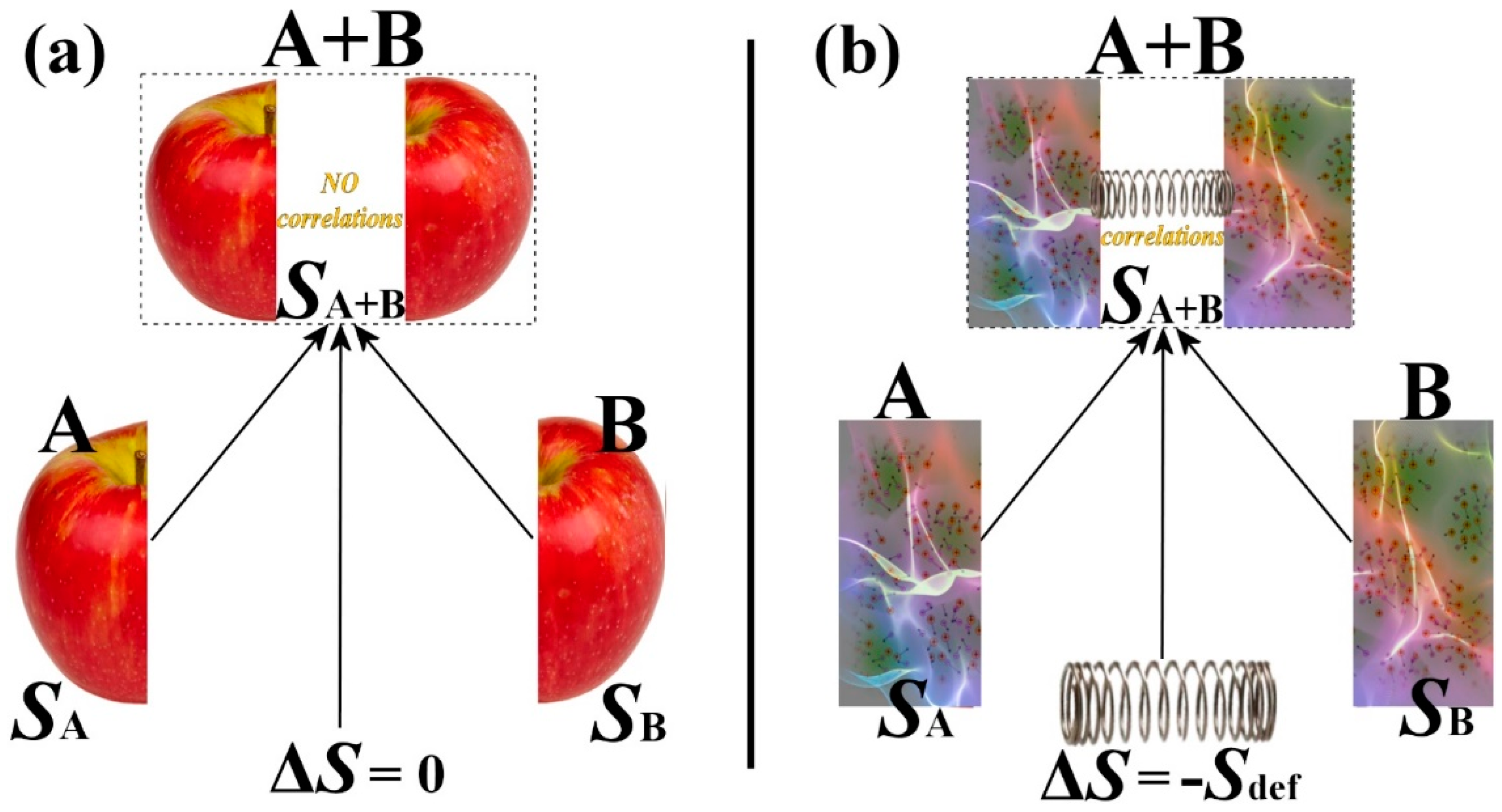
Entropy | Free Full-Text | Thermodynamic Definitions of Temperature and Kappa and Introduction of the Entropy Defect

Molar Heat Capacity at Constant Volume for Isobutane at Temperatures from (114 to 345) K and at Pressures to 35 MPa | Journal of Chemical & Engineering Data

The molar specific heat at constant pressure of an ideal gas is (7/2) R. the ratio of specific heat at constant pressure to that at constant volume is

For an ideal gas the molar heat capacity varies as C = CV + 3aT^2 . Find the equation of the process in the variables (T,V) where a is a constant.

For an ideal gas the molar heat capacity varies as C = CV + 3aT^2 . Find the equation of the process in the variables (T,V) where a is a constant.

mu PT$$ statistical ensemble: systems with fluctuating energy, particle number, and volume | Scientific Reports
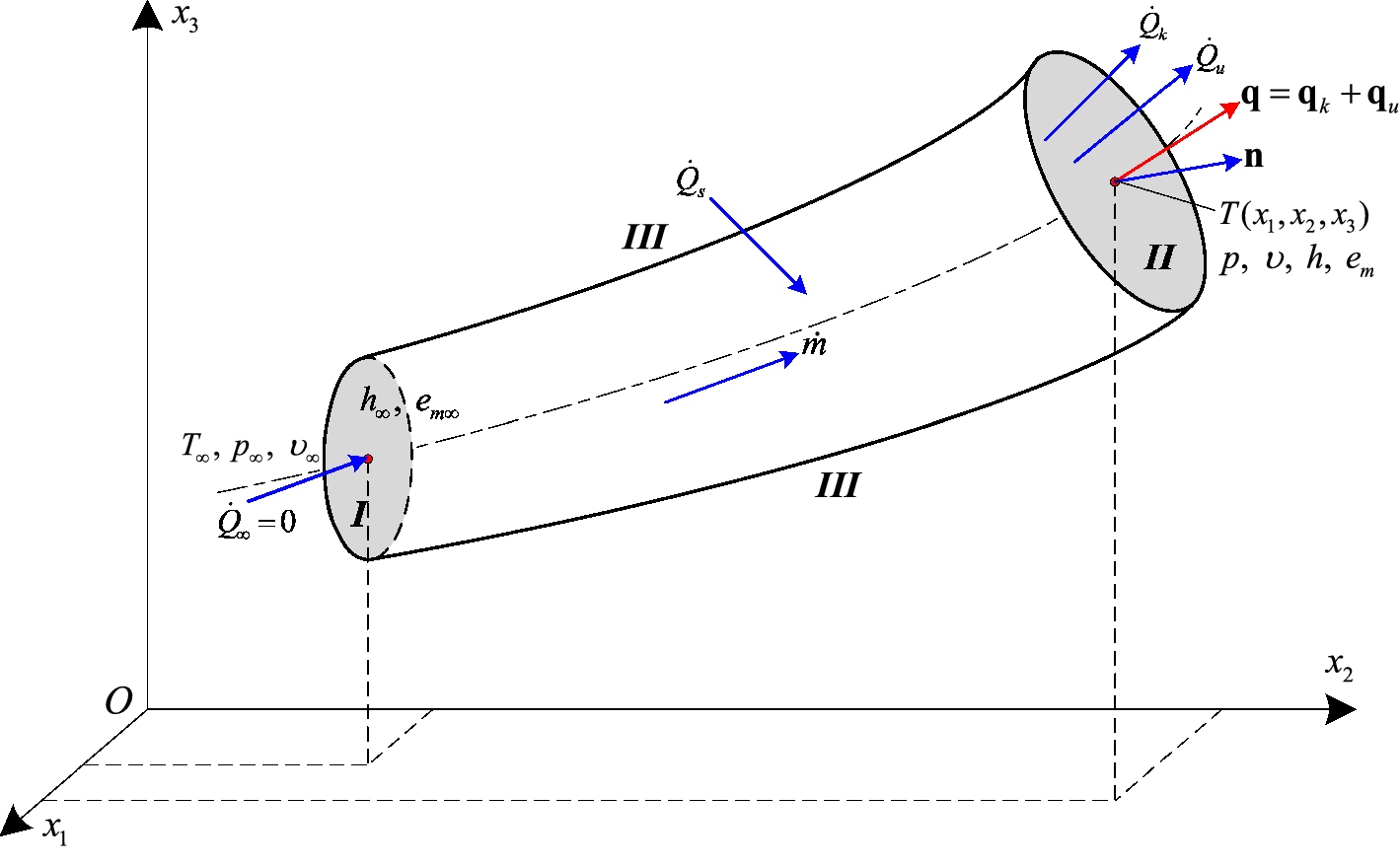
Derivation of unifying formulae for convective heat transfer in compressible flow fields | Scientific Reports

The specific heat capacity of a monoatomic gas for the process TV^2 = constant will be (R = gas constant).